Formation and decay of negative ion states up to 11 eV above the ionization energy of the nanofabrication precursor HFeCo3(CO)12†
Abstract
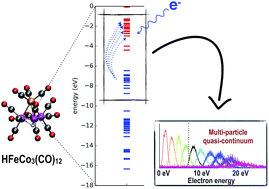
In single electron collisions with the heteronuclear metal carbonyl compound HFeCo3(CO)12 we observe the formation of long-lived negative ion states up to about 20 eV, 11 eV above its ionization energy. These transient negative ions (TNIs) relax through dissociation (dissociative electron attachment, DEA), losing up to all 12 CO ligands, demonstrating their resilience towards reemission of the captured electron – even at such very high energies. This is unique in DEA and we hypothesize that this phenomenon is rooted in the orbital structure enabling a scaffold of multi-particle, electronically excited resonances. We support this with calculated MO-diagrams revealing dense bands of energy levels near the HOMO–LUMO gap. HFeCo3(CO)12 is a promising focused electron beam induced deposition (FEBID) precursor and we argue that its unusual DEA behavior relates to its exceptional performance in FEBID. This may be general to a class of molecules with high potential for nano-fabrication by FEBID.


 Please wait while we load your content...
Please wait while we load your content...