Kinetic resolution of racemic 2-substituted 1,2-dihydroquinolines via asymmetric Cu-catalyzed borylation†
Abstract
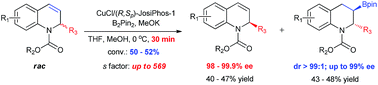
A highly efficient kinetic resolution of racemic 2-substituted 1,2-dihydroquinolines via asymmetric Cu-catalyzed borylation has been realized for the first time. Under mild conditions, a variety of chiral 3-boryl-1,2,3,4-tetrahydroquinolines containing two vicinal stereogenic centers as well as the recovered 2-substituted 1,2-dihydroquinolines were afforded after 30 minutes in high yields with up to 99% ee (dr > 99 : 1) and over 98% ee values, respectively, corresponding to kinetic selectivity factors of up to 569. Moreover, this protocol was successfully applied to the asymmetric synthesis of a selective estrogen receptor modulator.


 Please wait while we load your content...
Please wait while we load your content...