Metal-free direct alkylation of unfunctionalized allylic/benzylic sp3 C–H bonds via photoredox induced radical cation deprotonation†
Abstract
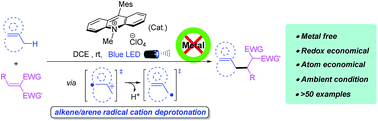
Despite notable recent efforts, a catalytic and convenient strategy for the direct alkylation of unactivated allylic or benzylic sp3 C–H bonds remains a formidable challenge facing the synthesis community. We herein report an unprecedented allylic/benzylic alkylation using only an organo-photoredox catalyst, which enables coupling of a broad scope of alkenes/arenes and electron-deficient alkenes in an atom- and redox-economic manner. A photoredox induced alkene/arene radical cation deprotonation is proposed to smoothly generate the key allylic and benzylic radical intermediates. It represents the first C–C bond formation via radical cation deprotonation under visible light conditions. The resulting products can be easily scaled up and directly converted to γ,δ-unsaturated or α,β-diaryl-acids, -esters, -amides, -pyrazoles, -isoxazoles, as well as lactones, which enables this mild and selective sp3 C–H alkylation to rapidly access complex bioactive molecules.
- This article is part of the themed collection: Most downloaded articles of 2017: Energy and Catalysis


 Please wait while we load your content...
Please wait while we load your content...