Electrocatalytic synthesis of ammonia by surface proton hopping†
Abstract
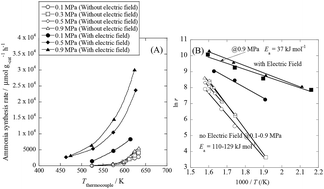
Highly efficient ammonia synthesis at a low temperature is desirable for future energy and material sources. We accomplished efficient electrocatalytic low-temperature ammonia synthesis with the highest yield ever reported. The maximum ammonia synthesis rate was 30 099 μmol gcat−1 h−1 over a 9.9 wt% Cs/5.0 wt% Ru/SrZrO3 catalyst, which is a very high rate. Proton hopping on the surface of the heterogeneous catalyst played an important role in the reaction, revealed by in situ IR measurements. Hopping protons activate N2 even at low temperatures, and they moderate the harsh reaction condition requirements. Application of an electric field to the catalyst resulted in a drastic decrease in the apparent activation energy from 121 kJ mol−1 to 37 kJ mol−1. N2 dissociative adsorption is markedly promoted by the application of the electric field, as evidenced by DFT calculations. The process described herein opens the door for small-scale, on-demand ammonia synthesis.


 Please wait while we load your content...
Please wait while we load your content...