Metalloradical activation of α-formyldiazoacetates for the catalytic asymmetric radical cyclopropanation of alkenes†
Abstract
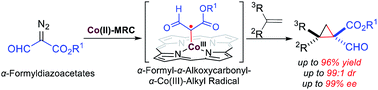
For the first time, α-formyldiazoacetates have been successfully applied for the asymmetric cyclopropanation of alkenes via Co(II)-based metalloradical catalysis. The cobalt(II) complex of the D2-symmetric chiral amidoporphyrin [Co(3,5-DitBu-ChenPhyrin)] is an effective metalloradical catalyst that can activate α-formyldiazoacetates to cyclopropanate both aromatic and aliphatic olefins with varied electronic properties, affording the synthetically useful 1,1-cyclopropaneformylesters in high yields with both high diastereo- and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...