A convergent synthesis of 1,3,4-oxadiazoles from acyl hydrazides under semiaqueous conditions†
Abstract
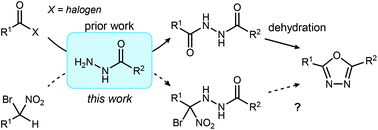
The 1,3,4-oxadiazole is an aromatic heterocycle valued for its low-lipophilicity in drug development. Substituents at the 2- and/or 5-positions can modulate the heterocycle's electronic and hydrogen bond-accepting capability, while exploiting its use as a carbonyl bioisostere. A new approach to 1,3,4-oxadiazoles is described wherein α-bromo nitroalkanes are coupled to acyl hydrazides to deliver the 2,5-disubstituted oxadiazole directly, avoiding a 1,2-diacyl hydrazide intermediate. Access to new building blocks of oxadiazole-substituted secondary amines is improved by leveraging chiral α-bromo nitroalkane or amino acid hydrazide substrates. The non-dehydrative conditions for oxadiazole synthesis are particularly notable, in contrast to alternatives reliant on highly oxophilic reagents to effect cyclization of unsymmetrical 1,2-diacyl hydrazides. The mild conditions are punctuated by the straightforward removal of co-products by a standard aqueous wash.


 Please wait while we load your content...
Please wait while we load your content...