Total synthesis of aristolactam alkaloids via synergistic C–H bond activation and dehydro-Diels–Alder reactions†
Abstract
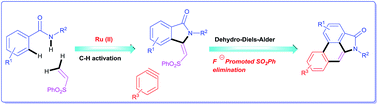
A concise total synthesis of aristolactam alkaloids by a synergistic combination of C–H bond activation and dehydro-Diels–Alder reactions is described. To achieve the synthesis two new synthetic methodologies, namely the oxidative cyclization of benzamides with vinyl sulfone leading to 3-methyleneisoindolin-1-ones via a ruthenium-catalyzed C–H bond activation, and a dehydro-Diels–Alder reaction followed by the fluoride ion mediated desulfonylation of 3-methyleneisoindolin-1-ones with benzynes, were developed. The method presented allows the opportunity for the construction of all the rings of aristolactams from easily available starting materials.


 Please wait while we load your content...
Please wait while we load your content...