The protecting-group free selective 3′-functionalization of nucleosides†
Abstract
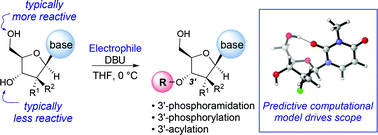
The direct and chemoselective 3′-phosphoramidation, phosphorylation and acylation of nucleosides are described. Upon the discovery of a novel 3′-phosphorylamidation of therapeutic nucleoside analogues with DBU, we explored the mechanism of this rare selectivity through a combination of NMR spectroscopy and computational studies. The NMR and computational findings allowed us to develop a predictive computational model that accurately assesses the potential for 3′-functionalization for a broad range of nucleosides and nucleoside mimetics. The synthetic utility of this model was exemplified by demonstration on a broad scope of nucleosides and electrophiles yielding targets that were previously only accessible via a protection/deprotection sequence or an enzymatic approach.


 Please wait while we load your content...
Please wait while we load your content...