Molecular catalysis at polarized interfaces created by ferroelectric BaTiO3†
Abstract
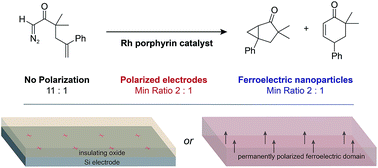
The local environment at polarized solid–liquid interfaces provides a unique medium for chemical reactions that could be exploited to control the selectivity of non-faradaic reactions. Polarized interfaces are commonly prepared by applying a voltage to an electrode in an electrolyte solution, but it is challenging to achieve high surface charge densities while suppressing faradaic reactions. Ferroelectric materials have permanent surface charge densities that arise from the dipole moments of ferroelectric domains and can be used to create polarized solid–liquid interfaces without applying a voltage. We studied the effects of ferroelectric oxides on the selectivity of a Rh porphyrin-catalyzed carbene rearrangement. The addition of ferroelectric BaTiO3 nanoparticles to the reaction solution changed the product ratio in the same direction and by a similar magnitude as performing the reaction at an electrode–electrolyte interface polarized by a voltage. The results demonstrate that colloidal suspensions of BaTiO3 nanoparticles act as a dispersible polarized interface that can influence the selectivity of non-faradaic reactions.


 Please wait while we load your content...
Please wait while we load your content...