Synthesis and reactivity of a ruthenocene-type complex bearing an aromatic π-ligand with the heaviest group 14 element†
Abstract
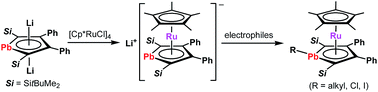
An anionic ruthenocene derived from a dilithioplumbole complex was prepared. In the complex, the plumbole ligand coordinates a ruthenium atom in an η5-fashion, similar to the cyclopentadienyl ligand in ferrocene. The ruthenocene that has the aromatic π-ligand with the heaviest group 14 element reacted with electrophiles to afford the plumbole complexes wherein the plumbole ligands show deviation from planarity, in contrast to the planar plumbole ring in the anionic ruthenocene. The bent angles of the plumbole ligands are dependent on the substituents on the lead atoms. Cyclic voltammetry measurements revealed that the plumbole complexes are oxidized more easily than the corresponding stannole complexes.


 Please wait while we load your content...
Please wait while we load your content...