Postsynthetic ionization of an imidazole-containing metal–organic framework for the cycloaddition of carbon dioxide and epoxides†
Abstract
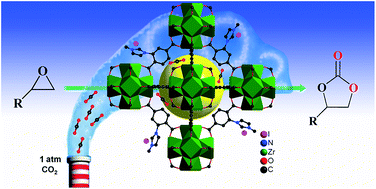
A bifunctional imidazolium functionalized zirconium metal–organic framework (Zr-MOF), (I−)Meim-UiO-66 (2), was successfully prepared from the imidazole-containing Zr-MOF Im-UiO-66 (1) by a post-synthetic modification (PSM) method. It was found that the crystal size and pore features of the imidazole-containing 1 could be tuned at the nanoscale. The bifunctional MOF 2, containing Brønsted acid sites and iodide ions, was shown to be an efficient and recyclable heterogeneous catalyst for the cycloaddition of carbon dioxide (CO2) with epoxides, without the use of any co-catalyst, at ambient pressure. The solvent-free synthesis of the cyclic carbonate from CO2 and an epoxide was monitored by in situ Fourier transform infrared spectroscopy (FT-IR) and an acid/base synergistic catalysis mechanism was proposed. We hope that our strategy provides an effective approach for the introduction of functional N-heterocyclic groups into MOFs for potential applications.
- This article is part of the themed collection: Most downloaded articles of 2017: Energy and Catalysis

 Please wait while we load your content...
Please wait while we load your content...