A new class of Cu/ZnO catalysts derived from zincian georgeite precursors prepared by co-precipitation†
Abstract
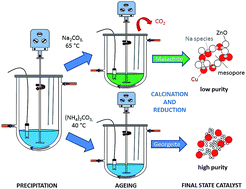
Zincian georgeite, an amorphous copper–zinc hydroxycarbonate, has been prepared by co-precipitation using acetate salts and ammonium carbonate. Incorporation of zinc into the georgeite phase and mild ageing conditions inhibits crystallisation into zincian malachite or aurichalcite. This zincian georgeite precursor was used to prepare a Cu/ZnO catalyst, which exhibits a superior performance to a zincian malachite derived catalyst for methanol synthesis and the low temperature water–gas shift (LTS) reaction. Furthermore, the enhanced LTS activity and stability in comparison to that of a commercial Cu/ZnO/Al2O3 catalyst, indicates that the addition of alumina as a stabiliser may not be required for the zincian georgeite derived Cu/ZnO catalyst. The enhanced performance is partly attributed to the exclusion of alkali metals from the synthesis procedure, which are known to act as catalyst poisons. The effect of residual sodium on the microstructural properties of the catalyst precursor was investigated further from preparations using sodium carbonate.


 Please wait while we load your content...
Please wait while we load your content...