Accessing human selenoproteins through chemical protein synthesis†
Abstract
The human body contains 25 selenoproteins, which contain in their sequence the twenty-first encoded amino acid, selenocysteine. About a dozen of these proteins remain functionally uncharacterized or poorly studied. Challenges in accessing these selenoproteins using traditional recombinant expressions have prevented biological characterization thus far. Chemical protein synthesis has the potential to overcome these hurdles. Here we report the first total chemical syntheses of two human selenoproteins, selenoprotein M (SELM) and selenoprotein W (SELW). The synthesis of the more challenging protein SELM was enabled using recent advances in the field of selenocysteine chemistry. This approach allows the preparation of selenoproteins in milligram quantities and in homogenous form, which should open new horizons for future studies to pursue a fuller biological understanding of their role in health and disease.
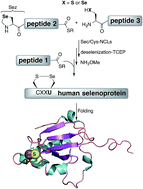
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST

 Please wait while we load your content...
Please wait while we load your content...