Green- to far-red-emitting fluorogenic tetrazine probes – synthetic access and no-wash protein imaging inside living cells†
Abstract
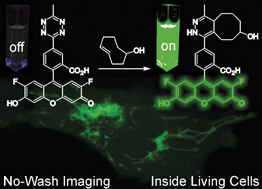
Fluorogenic probes for bioorthogonal labeling chemistry are highly beneficial to reduce background signal in fluorescence microscopy imaging. 1,2,4,5-Tetrazines are known substrates for the bioorthogonal inverse electron demand Diels–Alder reaction (DAinv) and tetrazine substituted fluorophores can exhibit fluorogenic properties. Herein, we report the synthesis of a palette of novel fluorogenic tetrazine dyes derived from widely-used fluorophores that cover the entire emission range from green to far-red. We demonstrate the power of the new fluorogenic probes in fixed and live cell labeling experiments and present the first example of intracellular live cell protein imaging using tetrazine-based probes under no-wash conditions.
- This article is part of the themed collection: Most downloaded articles of 2017: Analytical, Biological and Medicinal Chemistry

 Please wait while we load your content...
Please wait while we load your content...