The structural assembly switch of cell division protein FtsZ probed with fluorescent allosteric inhibitors†
Abstract
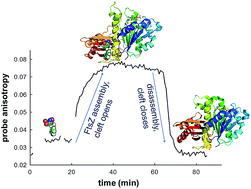
FtsZ is a widely conserved tubulin-like GTPase that directs bacterial cell division and a new target for antibiotic discovery. This protein assembly machine cooperatively polymerizes forming single-stranded filaments, by means of self-switching between inactive and actively associating monomer conformations. The structural switch mechanism was proposed to involve a movement of the C-terminal and N-terminal FtsZ domains, opening a cleft between them, allosterically coupled to the formation of a tight association interface between consecutive subunits along the filament. The effective antibacterial benzamide PC190723 binds into the open interdomain cleft and stabilizes FtsZ filaments, thus impairing correct formation of the FtsZ ring for cell division. We have designed fluorescent analogs of PC190723 to probe the FtsZ structural assembly switch. Among them, nitrobenzoxadiazole probes specifically bind to assembled FtsZ rather than to monomers. Probes with several spacer lengths between the fluorophore and benzamide moieties suggest a binding site extension along the interdomain cleft. These probes label FtsZ rings of live Bacillus subtilis and Staphylococcus aureus, without apparently modifying normal cell morphology and growth, but at high concentrations they induce impaired bacterial division phenotypes typical of benzamide antibacterials. During the FtsZ assembly–disassembly process, the fluorescence anisotropy of the probes changes upon binding and dissociating from FtsZ, thus reporting open and closed FtsZ interdomain clefts. Our results demonstrate the structural mechanism of the FtsZ assembly switch, and suggest that the probes bind into the open clefts in cellular FtsZ polymers preferably to unassembled FtsZ in the bacterial cytosol.


 Please wait while we load your content...
Please wait while we load your content...