A full set of iridium(iv) pyridine-alkoxide stereoisomers: highly geometry-dependent redox properties†
Abstract
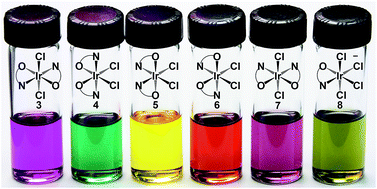
We introduce and characterize the complete set of possible isomers of IrIV(pyalk)2Cl2 (pyalk = 2-(pyridin-2-yl)propan-2-oate), providing valuable insights on the properties of Ir(IV) species. The pyridine alkoxide ligand strongly stabilizes high oxidation states, essential to accessing the catalytically relevant Ir(IV) state, and results in robust complexes that can be handled under ambient conditions, even permitting chromatographic separation. The redox properties are isomer-dependent, spanning a 300 mV range, rationalized with ligand-field theory and DFT calculations. The reported complexes exhibit very high kinetic inertness against isomerization, despite highly disparate predicted thermodynamic stabilities, presenting a unique opportunity to study all five possible isomeric complexes with the same ligand set.

 Please wait while we load your content...
Please wait while we load your content...