A quantitative mechanistic PK/PD model directly connects Btk target engagement and in vivo efficacy†
Abstract
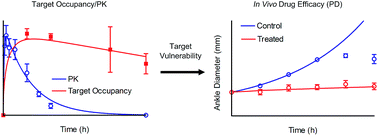
Correlating target engagement with in vivo drug activity remains a central challenge in efforts to improve the efficiency of drug discovery. Previously we described a mechanistic pharmacokinetic–pharmacodynamic (PK/PD) model that used drug–target binding kinetics to successfully predict the in vivo efficacy of antibacterial compounds in models of Pseudomonas aeruginosa and Staphylococcus aureus infection. In the present work we extend this model to quantitatively correlate the engagement of Bruton's tyrosine kinase (Btk) by the covalent inhibitor CC-292 with the ability of this compound to reduce ankle swelling in an animal model of arthritis. The modeling studies include the rate of Btk turnover and reveal the vulnerability of Btk to engagement by CC-292.


 Please wait while we load your content...
Please wait while we load your content...