Porous dendritic copper: an electrocatalyst for highly selective CO2 reduction to formate in water/ionic liquid electrolyte†
Abstract
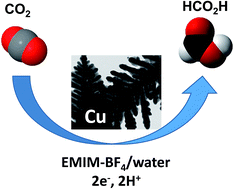
Copper is currently extensively studied because it provides promising electrodes for carbon dioxide electroreduction. The original combination, reported here, of a nanostructured porous dendritic Cu-based material, characterized by electron microcopy (SEM, TEM) and X-ray diffraction methods, and a water/ionic liquid mixture as the solvent, contributing to CO2 solubilization and activation, results in a remarkably efficient (large current densities at low overpotentials), stable and selective (large faradic yields) electrocatalytic system for the conversion of CO2 into formic acid, a product with a variety of uses. These results provide new directions for the further improvement of Cu electrodes.
- This article is part of the themed collections: Most downloaded articles of 2017: Energy and Catalysis, ISACS17: Challenges in Chemical Renewable Energy and Global Energy Challenges: Fossil Fuels

 Please wait while we load your content...
Please wait while we load your content...