Sequential catalysis: exploiting a single rhodium(i) catalyst to promote an alkyne hydroacylation–aryl boronic acid conjugate addition sequence†
Abstract
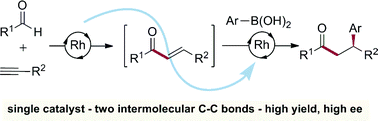
We demonstrate that a single Rh(I) complex can promote two mechanistically distinct C–C bond-forming reactions – alkyne hydroacylation and aryl boronic acid conjugate addition – to deliver substituted ketone products from the controlled assembly of three readily available fragments. This is a rare example of a Rh(I)/Rh(III) cycle and a redox neutral Rh(I) cycle being promoted by a single catalyst. The process is broad in scope, allowing significant variation of all three reaction components. Incorporation of an enantiomerically pure bis-phosphine ligand renders the process enantioselective. Superior levels of enantioselectivity (up to >99% ee) can be achieved from using a two catalyst system, whereby two Rh(I) complexes, one incorporating an achiral bis-phosphine ligand and the second a chiral diene ligand, are introduced at the start of the reaction sequence.


 Please wait while we load your content...
Please wait while we load your content...