Catalysis in flow: O2 effect on the catalytic activity of Ru(OH)x/γ-Al2O3 during the aerobic oxidation of an alcohol†
Abstract
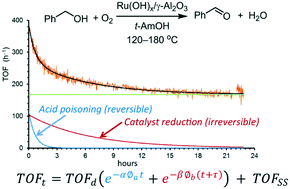
Changes in the turnover frequency (TOF) of Ru(OH)x/γ-Al2O3 during aerobic oxidation of benzyl alcohol in a plug flow differential reactor were monitored online using an inline FTIR instrument over extended periods of time (up to 72 h). A new equation for catalyst deactivation is derived, to account for the different lengths of time the catalyst is exposed to the reactants (benzyl alcohol and O2). Catalyst activity and stability are dependent on the amount of O2 in the system; catalyst deactivation can be attributed to two simultaneous processes: a fast and reversible inhibition by the benzoic acid side product and a slower and irreversible loss of catalytic sites due to reduction of Ru. At steady state, the reaction rate is zero-order in benzyl alcohol and partial positive order in O2. Finally, the suitability of a packed-bed (integral) reactor for the reaction is discussed.
- This article is part of the themed collection: Celebrating Excellence in Research: 100 Women of Chemistry


 Please wait while we load your content...
Please wait while we load your content...