Assessing inter- and intramolecular continuous-flow strategies towards methylphenidate (Ritalin) hydrochloride†
Abstract
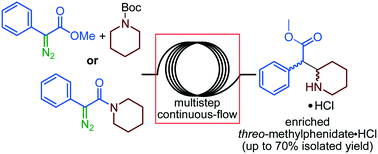
The batch-to-flow translation of inter- and intramolecular strategies for the diastereoselective preparation of the active pharmaceutical ingredient threo-methylphenidate hydrochloride is presented. Both inter- and intramolecular strategies imply the telescoping of multiple processing steps and the generation of unstable diazo species under continuous-flow conditions. The intermolecular strategy relies on an unprecedented continuous-flow Rh-catalyzed intermolecular C–H carbene insertion, providing enriched threo-N-Boc methylphenidate in 38% or 19% isolated yield according to sequential or fully telescoped processes, respectively. Quantitative Boc-deprotection is carried out off-line. The intramolecular strategy relies on a continuous-flow thermal intramolecular C–H carbene insertion, providing enriched threo-methylphenidate hydrochloride in 70% isolated yield. A continuous-flow photochemical alternative is also presented. The critical step of the most promising intramolecular strategy is implemented on the mesoscale in a pilot-scale continuous-flow reactor.
- This article is part of the themed collection: Reaction Chemistry & Engineering Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...