Environmentally benign and diastereoselective synthesis of 2,4,5-trisubstituted-2-imidazolines†
Abstract
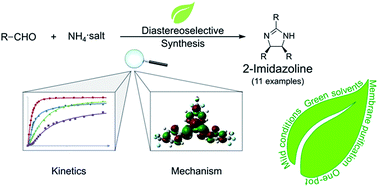
2-Imidazolines are heterocycles with a wide range of applications in biomedical science, pharmaceuticals, surfactants and catalysis. The 2,4,5-trisubstituted derivatives are conventionally prepared through the reaction of aromatic aldehydes and ammonia, requiring harsh conditions and toxic solvents. Herein, we report a sustainable, one-pot synthetic route to obtain cis or trans diastereomers of 2,4,5-trisubstituted-2-imidazolines. The reaction parameters, such as the ammonia source, the substituents of the aromatic aldehydes and the effect of temperature and solvent, were systematically explored. The synthesis was attempted in sixteen different green solvents. Reaction kinetics and quantum mechanical studies (DFT B3LYP/6-31G(d,p)) revealed the elementary steps of the cyclisation and refuted two common speculations. The limitations of the synthesis were explored through varying the p-, o-, and m-substituents on the aldehyde as well as employing heterocyclic and non-aromatic derivatives. Ten out of the twelve products synthesised in this work can be solely purified by crystallisation, without the need for extraction or chromatography. Owing to the bulky nature of the products, the applicability of membrane-based purification was demonstrated to further improve the sustainability of the synthesis.
- This article is part of the themed collection: Sustainable synthesis


 Please wait while we load your content...
Please wait while we load your content...