Control of the molecular packing of chloroboron(iii) and fluoroboron(iii) subnaphthalocyanines by designing peripheral substituents†
Abstract
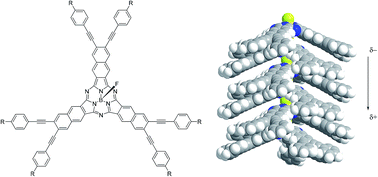
Chloroboron(III) and fluoroboron(III) hexa(1-alkynyl)- and hexa(2-arylethynyl)subnaphthalocyanines with a large dipole moment were prepared by Sonogashira coupling of hexaiodosubnaphthalocyanines with substituted acetylenes. Introduction of butyl or longer alkyl groups via ethynylene linkages on the periphery resulted in lower melting points and higher solubility in dichloromethane. X-ray diffraction (XRD) patterns of the cast film indicated that hexa(2-(4-hexylphenyl)ethynyl)- and hexa(2-(4-hexyloxyphenyl)ethynyl)subnaphthalocyanines with a B–F bond are packed in a discotic hexagonal columnar phase with lattice constants a = 59–64 Å and stacking distance c = 4.7–4.8 Å. The Q-band in visible spectra of the thin film of these B–F derivatives was blue-shifted, supporting the formation of H-aggregate in stacked columns. Polarized optical microscopy showed that these subnaphthalocyanines with a B–F bond exhibited a mesophase at 180 °C. XRD of these subnaphthalocyanines at 180 °C confirms a two dimensional hexagonal phase. XRD of the corresponding derivatives with a B–Cl bond showed that they were either amorphous or less crystalline. We suggest that the fluorine atom in the B–F group can fit into the cleft of cone-shaped subnaphthalocyanine, owing to the smaller van der Waals radius of F than Cl, to stabilize the columnar packing structure.
- This article is part of the themed collection: Editors' Collection: Phthalocyanines


 Please wait while we load your content...
Please wait while we load your content...