Promotion effect of water in catalytic fireplace soot oxidation over silver and platinum†
Abstract
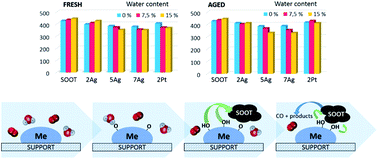
Fireplaces are one of the largest sources of soot emissions. One possible approach to reduce wood-based soot emission is to use catalysts that have been designed for fireplace applications. This study compares the activities of silver and platinum catalysts in the oxidation of real fireplace soot in different gas atmospheres. Another goal is to examine the effects of water on the catalyst activity depending on the moisture content in the reaction gas. The study shows that the gas feed composition affects the performance of the catalyst. Unlike platinum, the silver catalyst directly oxidizes the soot in the presence of air and NO. The addition of water into the air promotes the soot oxidation activity of both the silver and platinum catalysts. The catalysts improve their activity with the increase in water content. For both the silver and platinum catalysts, the partial dissociation of water with oxygen and the formation of hydroxyls are promoted, which oxidize soot. Thermal ageing shows that silver has a higher thermal stability and retains its oxidation activity for longer than platinum under all of the applied conditions. Silver is a more suitable option for the catalytic oxidation of soot emission of fireplaces.


 Please wait while we load your content...
Please wait while we load your content...