Tri- and hexanuclear heterometallic Ni(ii)–M(ii) (M = Ca, Sr and Ba) bis(salamo)-type complexes: synthesis, structure and fluorescence properties†
Abstract
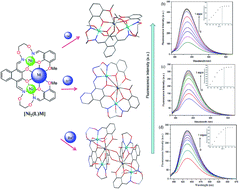
Three heterometallic Ni(II)–M(II) (M = Ca, Sr and Ba) complexes, two discrete heterotrinuclear complexes [Ni2(L)Ca(OAc)2(CH3OH)2]·2C2H5OH·2CHCl3 (1) and [Ni2(L)Sr(OAc)2(CH3OH)2]·2CH3OH·2CH2Cl2 (2) and a discrete heterohexanuclear dimer [Ni2(L)Ba(OAc)2(CH3OH)2(H2O)]2·2CH3OH (3), were synthesized with a naphthalenediol-based acyclic bis(salamo)-type ligand (H4L), and characterized by elemental analyses, IR, UV-vis spectra, fluorescence spectra and X-ray crystallography. The heterometallic complexes were acquired by the reaction of H4L with 2 equiv. of Ni(OAc)2·4H2O and 1 equiv. of M(OAc)2 (M = Ca, Sr and Ba). The crystal structures of complexes 1–3 have been determined by single-crystal X-ray diffractions. Owing to the different nature of the N2O2 and O6 sites of the ligand H4L, the introduction of two different metal(II) atoms to the site-selective moiety, leads to the two Ni(II) atoms occupied both the N2O2 sites, an alkaline earth metal atom occupied the O6 site of the ligand (L)4− unit, respectively. Furthermore, the fluorescence properties have been discussed.
- This article is part of the themed collection: 2017-2018 Top Cited Research from China


 Please wait while we load your content...
Please wait while we load your content...