In silico studies of solvated F19W amyloid β (11–40) trimer†
Abstract
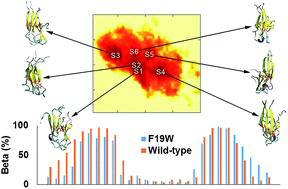
Alzheimer's disease (AD) is associated with the oligomerization and/or fibrillation of amyloid beta (Aβ) peptides, which cause damage to brain cells. Aβ oligomers and fibrils contain hydrophobic cores formed with parallel beta sheets. Mutations of F19, a residue in the hydrophobic core of Aβ peptides, slow down their aggregation process but do not alter the overall structure of the resulting fibrils. However, the effects of F19 mutations on the toxic Aβ oligomers have not been elucidated. We studied the F19W mutant of the 11–40 truncated Aβ trimer (F19W 3Aβ11–40) using replica exchange molecular dynamics (REMD) simulations. While most structural terms do not change significantly, critical polar contacts decrease by 20%, and notably, RMSD almost doubles upon F19W mutation. Six minima were found in the free energy surface of F19W 3Aβ11–40, which have lower energy barriers (by ∼1 kJ mol−1) and significantly lower total population (∼20%) compared to those of the three minima found for 3Aβ11–40 (∼60%). The binding free energy between constituting chains of the mutant trimer increases by ∼28 kcal mol−1 but fluctuates significantly (±27.1 kcal mol−1). Our results indicate that while the hydrophobic core of amyloid beta peptide is capable of adapting to structural changes, F19W mutation results in a significantly more flexible trimer. The more flexible F19W mutant oligomers would require more time to self-assemble into fibrils. Our results contribute to a better understanding of the behavior of Aβ peptides and their oligomerization/aggregation process, which is necessary to understand AD pathogenesis.


 Please wait while we load your content...
Please wait while we load your content...