Dependence of electrochemical properties of spinel LiMn2O4 on Li2CO3 with micro-flaky, micro-flower and nanorod morphologies†
Abstract
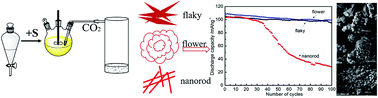
Herein, the dependence of spinel LiMn2O4 on Li2CO3 with micro-flaky, micro-flower and nanorod morphologies is investigated. The results show that the as-synthesized LiMn2O4 with micron sized Li2CO3 as raw materials have a much higher discharge capacity than that of the one prepared with nano sized Li2CO3. It delivers an initial charge capacity of 110.1, 105.2 and 104.9 mA h g−1 followed by a discharge capacity of 109.1, 103.9 and 104.2 mA h g−1 with the micro-flower, nanorod and micro-flaky Li2CO3 morphologies, respectively, at room temperature (about 99% of the charge capacity is discharged). The smaller specific surface area is found in the spinel LiMn2O4 with micron sized Li2CO3, resulting in a better stable electrochemical performance in LiMn2O4 with micro-flower and micro-flaky Li2CO3. Their capacities are maintained at 99.2 mA h g−1 and 94.2 mA h g−1 after 100 cycles at 1C rate. The capacity retention was more than 90% at the 100th cycle with the micron-sized Li2CO3. Moreover, the as-synthesized spinel LiMn2O4 with micro-flower Li2CO3 retained more than 95% of its initial discharge capacity (92 mA h g−1) after 200 cycles at 2C rate. The cubic spinel structure was detected after 200 cycles of LiMn2O4 at 2C rate.


 Please wait while we load your content...
Please wait while we load your content...