Synthesis and insecticidal assessment of some innovative heterocycles incorporating a thiadiazole moiety against the cotton leafworm, Spodoptera littoralis†
Abstract
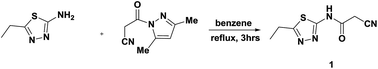
New 2-cyano-N-(5-ethyl-1,3,4-thiadiazol-2-yl)acetamide (1) was utilized as a versatile precursor for the synthesis of various heterocycles, such as pyrrole, pyridine, coumarin, thiazole, pyrido[2′,3′:3,4]pyrazolo[5,1-c]triazine, triazolo[5,1-c]triazine, aminopyrazole, thiophene, 1,3-dithiolane, triazolo[1,5-a]pyrimidine and benzo[d]imidazole derivatives. The newly synthesized compounds were identified by IR, MS, 1H NMR, 13C NMR, DEPT, H–H COSY, HMBC, and HSQC. Representative compounds of the synthesized products were examined and estimated as insecticidal agents against the cotton leafworm, Spodoptera littoralis.


 Please wait while we load your content...
Please wait while we load your content...