Interactions between sirtuins and fluorogenic small-molecule substrates offer insights into inhibitor design†
Abstract
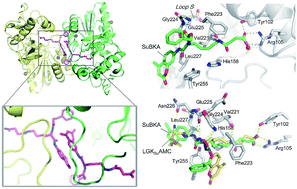
Sirtuins are nicotinamide adenine dinucleotide (NAD+)-dependent lysine deacylases regulating metabolism and stress responses and are involved in human pathologies such as neurodegeneration. In this study, four fluorogenic small-molecule substrates, i.e., acetyl-(AcBKA), crotonyl-(CrBKA), succinyl-(SuBKA), and myristoyl-(MyBKA)-containing substrates, were synthesized and tested against three representative sirtuin isoforms (i.e., SIRT2, SIRT5, and SIRT6). Enzyme kinetic results indicate that the fluorogenic small-molecule substrates have similar sirtuin-isoform preference as compared to peptide substrates. ITC analyses reveal that AcBKA or MyBKA binding to SIRT2 is mainly driven by entropy, whereas SuBKA binding to SIRT5 is driven by enthalpy. The SIRT5:SuBKA complex crystal structure reveals a new substrate-binding mode that is different from peptide substrate binding modes, but involves Tyr102, Arg105, and other catalytically important residues on Loop S; this indicates that SuBKA is desuccinylated by SIRT5 probably through the catalytic mechanism proposed for peptide substrates. The biophysical and structural results presented herein will provide thermodynamic insights and key pharmacophore features for the development of selective sirtuin isoform-specific inhibitors.


 Please wait while we load your content...
Please wait while we load your content...