Photodegradation of sulfadiazine catalyzed by p-benzoquinones and picric acid: application to charge transfer complexes†
Abstract
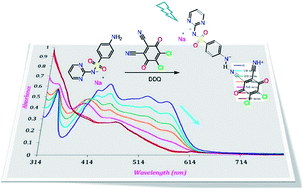
As the treatment of effluents containing the antibiotic drug sulfadiazine (SZ) is one of the challenging problems in the field of environmental chemistry, it is essential to determine the concentration of SZ by a rapid and accurate method and then find a suitable method to degrade the assayed products into harmless chemicals. The color of the charge transfer (CT) complexes developed from the reaction of SZ with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), chloranilic acid (CHL) and picric acid (PA) was used to determine the concentration of SZ at 528, 510 and 410 nm, respectively. The Lambert–Beer's law is obeyed in the ranges of 6.80–68.06, 13.61–136.12 and 6.80–27.22 μg mL−1 for DDQ, CHL and PA complexes. The photolysis of SZ → DDQ in presence of sodium nitrite at 256 nm leads to faster degradation of SZ compared with the control experiments. This was simply spectrophotometrically followed by a decrease in the intensity of the CT band. The effect of some additives such as oxalic acid, and hematite nano particles was studied. For comparison, other π-acceptor reagents such as CHL and PA were used. About 80% of SZ is degraded in 45 min upon the illumination of SZ → DDQ at 256 nm, whereas 90 min is required in the case of CHL and PA to attain the same degradation limit.


 Please wait while we load your content...
Please wait while we load your content...