Enhanced photocatalytic activity for degrading phenol in seawater by TiO2-based catalysts under weak light irradiation†
Abstract
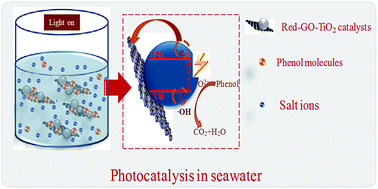
The seawater system is a typical salt water system. A catalyst should overcome the disturbance from the salt ions for an efficient photodegradation of organic pollutants in the seawater system. Commercial photocatalysts (P25) and La3+-doped SiO2–TiO2 prepared using adsorbed-layer nanoreactor synthesis (ALNS) were first used for photodegrading different initial concentrations of phenol in seawater under weak UV light irradiation. The weak adsorption capacities for phenol and the hydrophilic surfaces of the two photocatalysts could not overcome the disturbance of salt ions and thus showed low photocatalytic activities. Based on this, graphene oxide (GO) was used as a support to prepare TiO2 and La3+-doped TiO2 using ALNS. The solvothermal treatment with alcohol was used as a solvent for both TiO2 crystallization and GO reduction. Results showed that TiO2 nanoparticles with sizes <10 nm formed and distributed homogeneously on the reduced GO surface. The small size of the TiO2 particles and the decreased oxygenated functional groups on the GO surface both caused high separation efficiency of the photogenerated charge carriers, thereby increasing the photodegradation performance. The strong phenol adsorption of the photocatalyst was efficient enough to overcome the interference of salt ions and enhance the photodegradation efficiency in seawater. The activities of the two Red–GO–TiO2 catalysts were more than twice those of P25 and La3+-doped SiO2–TiO2. La3+ doping caused mixed crystals to form and increased the shallow trapping sites for charge carriers. Therefore, La3+ doping increases the photocatalytic activity of Red–GO–TiO2.


 Please wait while we load your content...
Please wait while we load your content...