Characterization and acid-catalysed depolymerization of condensed tannins derived from larch bark
Abstract
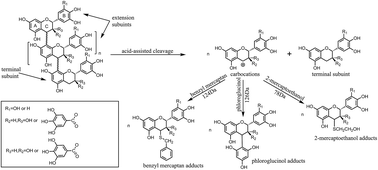
Condensed tannins from larch bark extracts are a natural renewable and eco-friendly material and are potential substitutes for phenolic petrochemicals. However, the wide application of tannin is restricted by its low reactivity. Therefore, the goal of this study was to enhance the reactivity of larch tannin by depolymerization and determine optimal reaction conditions. The structures of larch tannin and depolymerized larch tannin were characterized by Fourier transform infrared (FT-IR) spectroscopy, solid phase 13C-NMR and matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry. The thermal stability of larch tannin before and after depolymerization was evaluated by thermogravimetric analysis (TGA). The results indicated that the monomeric units of larch tannin were mainly composed of catechin/epicatechin, gallocatechin/epigallocatechin, catechin–gallocatechin esters, and stilbene glucosides. The presence of a catechin gallate dimer that had lost both gallic acid residues and a hydroxy group and a small amount of fisetinidin units were also observed. Additionally, a series of peaks corresponding to oligomers of larch tannin of up to 11 repeating units were observed from the MALDI-TOF MS data. Depolymerization treatment, especially using 2-mercaptoethanol as a nucleophilic reagent, was found to be beneficial to the enhancement of thermal stability. The optimization of the depolymerization reaction allowed the reaction to be completed in two hours, at 60 °C, using 2-mercaptoethanol as a nucleophile and 0.1 mol L−1 HCl. Many compounds of molecular weight less than 600 Da, mainly dimers and monomers, were obtained under these reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...