Improved bioavailability of curcumin in liposomes prepared using a pH-driven, organic solvent-free, easily scalable process
Abstract
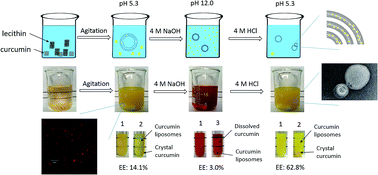
The poor water solubility and bioavailability of curcumin can be improved by encapsulating it into liposomes. However, the existing encapsulation technologies, such as the thin film method and the ethanol injection method, are complex and require the use of organic solvents. In this study, an organic solvent-free and easily scalable encapsulation technique was studied by utilizing the pH-dependent solubility properties of curcumin. Phospholipid was dissolved in water to form liposomes. Curcumin was deprotonated and dissolved under alkaline conditions and then encapsulated into the liposomes after acidification. Morphological observation and X-ray diffraction analysis confirmed that curcumin liposomes had been successfully prepared. Curcumin liposomes prepared by the pH-driven method were stable during storage. During in vitro digestion, curcumin liposomes prepared by the pH-driven method showed similar bioaccessibility to those prepared by the thin film method and higher bioaccessibility than those prepared by the ethanol injection method. The pH-driven method, which is organic solvent-free and easily scalable for industrial production, is thus a promising method for the preparation of curcumin liposomes.


 Please wait while we load your content...
Please wait while we load your content...