Development of contact-killing non-leaching antimicrobial guanidyl-functionalized polymers via click chemistry
Abstract
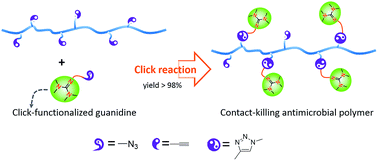
An expedient and efficient method applied to synthesize click-suitable penta-substituted guanidines at room temperature is hereby described. The guanidine containing polyurethanes (PU-TMGs) were prepared via click reaction (copper catalyzed alkyne-azide [3 + 2] cycloaddition, CuAAC), which was performed either before or after polymerization to incorporate the guanidine. The guanidine-functionalized polymers exhibited strong contact-killing antibacterial activity against both Gram-negative and Gram-positive bacteria. There is around 99.9% killing of S. aureus and 98.0% killing of E. coli when TMG weight percentage content in PU-TMG is around 1%. Covalent conjugation of the guanidine groups to the polymers prevents leaching. The conjugation also reduces cytotoxicity of the materials and preserves long-term antimicrobial activity.
- This article is part of the themed collection: Editors’ collection: Antimicrobial Polymers


 Please wait while we load your content...
Please wait while we load your content...