Synthesis and characterization of a high temperature thermosetting polyimide oligomer derived from a non-toxic, sustainable bisaniline
Abstract
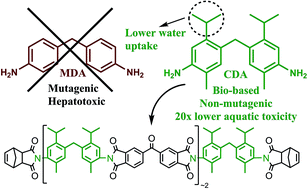
PMR-15 is a high temperature thermosetting polyimide oligomer widely used in aircraft engines, rocket casings, bushings, and bearings. Unfortunately, PMR-15 is prepared from the mutagenic and hepatotoxic compound methylene dianiline (MDA). To develop a safer alternative to PMR-15, an alternative thermosetting polyimide oligomer (PMR-PCy) was prepared from a less toxic bisaniline, 4,4′-methylenebis-(5-isopropyl-2-methylaniline) (CDA), derived from the renewable aromatic compound p-cymene. The thermoset network prepared from PMR-PCy had a glass transition temperature (Tg) of 323 °C as measured by differential scanning calorimetry (DSC), good thermo-oxidative stability, and water uptake of only 3% after immersion in boiling water for 24 h. The Quantitative Structure Activity Relationship (QSAR)-predicted low toxicity of CDA was confirmed by in vitro testing for mutagenicity, acute toxicity, and aquatic toxicity. CDA was found to be non-mutagenic in the Ames test, had a predicted LD50 of 725 mg kg−1 in rats, and an EC50 of 299.3 mg L−1. These results suggest that CDA is not acutely toxic to humans and has minimal to no aquatic toxicity. The combination of exceptional material properties coupled with the low toxicity of CDA suggest that PMR-PCy may be a viable non-toxic replacement for PMR-15 in a variety of aerospace applications.


 Please wait while we load your content...
Please wait while we load your content...