Urea-assisted synthesis of hydroxyapatite nanorods from naturally occurring impure apatite rocks for biomedical applications†
Abstract
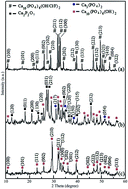
Hydroxyapatite (HA) nanoparticles are heavily used materials in biomedical applications. Therefore, identification of cheap and readily available raw-materials for the synthesis of HA nanoparticles is very important to fulfill the current demand. Herein, for the first time, we have developed a novel method to convert readily available, extensively distributed, naturally occurring apatites into nontoxic hydroxyapatite nanoparticles for biomedical applications. In this method, powdered apatite is digested and combusted to produce calcium phosphate nanoparticles and hydrothermally treated to convert them into high purity HA. HA nanoparticles are characterized using X-ray diffraction (XRD), Fourier Transform Infrared (FT-IR) spectroscopy, Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM). Synthesized HA nanoparticles are nontoxic according to the cytotoxicity results which confirm their potential usage in biomedical applications. Therefore, this method is very important to fulfill the current demand of HA nanoparticles and for value-addition to natural apatite.


 Please wait while we load your content...
Please wait while we load your content...