Degradation of one-side fully-chlorinated 1,2,3,4-tetrachloronaphthalene over Fe–Al composite oxides and its hypothesized reaction mechanism†
Abstract
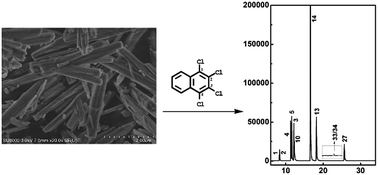
The degradation of 1,2,3,4-tetrachloronaphthalene (CN-27) featuring a one-side fully-chlorinated aromatic ring, was evaluated over three of the prepared rod-like Fe–Al composite oxides (FeAl-1, FeAl-5 and FeAl-10). The results showed that their reactive activities were in the order of FeAl-5 ≈ FeAl-10 ≫ FeAl-1, which could be attributed to their different pore structural properties and reactive sites caused by the different phase interaction between iron species and the γ-Al2O3. The generation of trichloronaphthalenes (1,2,3-TrCN and 1,2,4-TrCN, i.e. CN-13 and CN-14), dichloronaphthalenes (1,2-DiCN, 1,3-DiCN, 1,4-DiCN and 2,3-DiCN, i.e. CN-3, CN-4, CN-5 and CN-10) and monochloronaphthalenes (1-MoCN and 2-MoCN, i.e. CN-1 and CN-2) suggested the occurrence of successive hydrodechlorination reactions. The amount of CN-14 exceeded that of CN-13 from 71.5% to 77.7% across the three different systems, revealing the preferred occurrence of the first hydrodechlorination step at the β-position. This is dissimilar to the preference at the α-position observed during the dechlorination of octachloronaphthalene (CN-75) over micro/nano Fe3O4. The structural differences between one-side and two-side fully-chlorinated aromatic rings would have a pronounced impact on the reactivity of the chlorine substitution position. The major hydrodechlorination pathway was judged to be CN-27 → CN-14 → CN-4 → CN-2. Additionally, the detected 1,2,3,4,6-pentachloronaphthalene (CN-50) and 1,2,4,6/7-tetrachloronaphthalenes (CN-33/34) suggested the reverse chlorination reaction also happened while the hydrodechlorination reaction was occurring. The C–Cl bond dissociation energies (BDEs) of the parent and daughter polychlorinated naphthalene (PCN) congener were calculated using density functional theory (DFT), to achieve a deeper understanding of a different product yield distribution.


 Please wait while we load your content...
Please wait while we load your content...