An octanuclear Schiff-base complex with a Na2Ni6 core: structure, magnetism and DFT calculations†
Abstract
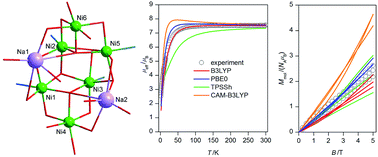
The octanuclear Na2Ni6 complex (Pr3NH)[Na2Ni6(L)4(Bza)5(HBza)(OH)2(ace)]·Et2O (1), where Pr3NH+ = the tripropylamonium cation, H2L = 2-[(E)-(2-hydroxybenzylidene)amino]phenol, HBza = benzoic acid and ace = acetone, was synthesized and characterized by the elemental analysis, FTIR spectroscopy, single crystal X-ray diffraction analysis, magnetic measurements and DFT calculations. All six NiII atoms are hexacoordinate with the {NiO6} or {NiNO5} chromophores forming two defective dicubane cores. The static magnetic data were fitted to the simplified spin Hamiltonian model which resulted in averaged ferromagnetic and antiferromagnetic exchange parameters J = +5.3 cm−1, and J = −9.2 cm−1, respectively, confirming the predominant role of the antiferromagnetic coupling in 1. The broken symmetry DFT method with various functionals (B3LYP, PBE0, TPPSh and CAM-B3LYP) was used to dissect information about magnetic coupling. As a result, the isotropic exchange parameters (Jab) derived by the PBE0 or B3LYP functionals seem to be the best to match the experimental magnetic data.


 Please wait while we load your content...
Please wait while we load your content...