Synergy of adsorption and photosensitization of graphene oxide for improved removal of organic pollutants†
Abstract
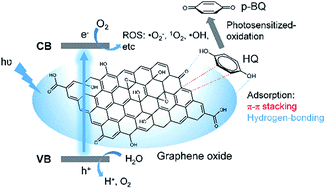
Due to the various functional oxygen-containing groups, graphene oxide (GO) has been frequently explored as sorbent for various metal ions and organic pollutants. However, the adsorption capacity of solely GO is limited. Therefore, compositing GO with other adsorbents to improve the adsorption capacity is often required. On the other hand, the oxygen functional groups enable GO for exciton generation, which has been harvested for photosensitized generation of reactive oxygen species (ROS). Photosensitization has been explored for advanced pollutant degradation. Here, the adsorption and photosensitization effect of GO was synergized for improving the pollutant removal performance. Hydroquinone (HQ) was taken as the model pollutant for the illustration of the synergic effect. GO could adsorb HQ, possibly due to π–π stacking and hydrogen-bonding interactions between GO and HQ. In the presence of white light irradiation (LED), the removal efficiency for HQ was greatly improved. Spectroscopic study indicated the improved removal efficiency could be ascribed to light irradiation-induced HQ oxidation. Further study showed that violet LEDs exhibited higher oxidation efficiency than white, blue, green, yellow, and red LEDs, since violet LEDs possess the largest spectra overlap with the absorption of GO. Via scavenger studies, the exact oxidants were identified as ˙OH, 1O2, and ˙O2−, which are generated from GO photosensitization. Another intriguing feature is that GO can be recycled in several runs of adsorption/photosensitization of pollutants. Moreover, the synergic adsorption/photosensitization feature of GO can be extended to remove a broad band of phenolic pollutants, demonstrating the universality of such strategy for improved pollutant removal.


 Please wait while we load your content...
Please wait while we load your content...