2,3-Disubstituted-1,4-naphthoquinones containing an arylamine with trifluoromethyl group: synthesis, biological evaluation, and computational study†
Abstract
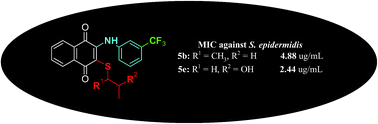
Antibacterial and antifungal organic compounds are becoming increasingly important for biomedical applications. This study deals with the synthesis, characterization of structures, in silico PASS prediction, and the discovery of antibacterial and antifungal properties based on new sulfanyl-1,4-naphthoquinone derivatives containing an arylamine with a trifluoromethyl group at different positions, which can be further applied in drug discovery and development. The in vitro antimicrobial potential of the newly synthesized compounds was evaluated in a panel of seven bacterial strains (three Gram-positive and four Gram-negative bacteria) and one yeast, with an additional study on antibiofilm activities. The compounds (5b and 5e) were identified as having strong antibacterial efficiency against the human-originated pathogen S. epidermidis, with minimal inhibitory concentration values (4.88 and 2.44 μg mL−1, respectively). The toxicity of both compounds (5b and 5e) was studied in detail to compare these compounds with Cefuroxime (a clinically proven drug). The antibacterial activity of the compound 5f was equal to that of Cefuroxime. Moreover, three compounds (5b, 5e, and 5f) exhibited excellent antibacterial activity, and 5b and 5e were two and four times more active than the reference antimicrobial compound (Cefuroxime), respectively. For this reason, these three compounds (5b, 5e, and 5f) are being considered as promising antibacterial agents. In addition, docking studies were used to better rationalize the action and prediction of the binding modes of these compounds.
- This article is part of the themed collection: Computational chemistry


 Please wait while we load your content...
Please wait while we load your content...