Diazepinium perchlorate: a neutral catalyst for mild, solvent-free acetylation of carbohydrates and other substances†
Abstract
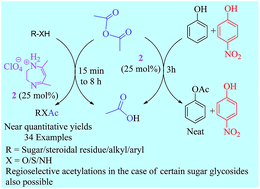
Diazepinium perchlorate, an essentially neutral organic salt possessing excellent stability, has been found to be well suited for the acetylation of free as well as partially protected sugars, phenols, thiophenols, thiols and other alcohols as well as amines. The diazepinium perchlorate-catalyzed acetylation is mild, organic and solvent-free and leaves acid sensitive protecting groups such as TBDMS/TBDPS/Tr ethers and isopropylidene/benzylidene acetals present on a substrate unaffected. Regioselective hydroxyl protection in partially protected carbohydrate derivatives/polyhydroxylic compounds was possible and was proved to be a convenient time-saving alternative to the conventional synthesis of such compounds. Easy preparation of the catalyst, mild reaction conditions and an environmentally benign protocol are some of the notable features of this reaction. The results obtained on the acetylation of phenols and thiophenols could be rationalized through their local nucleophilicity index obtained from DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...