Theoretical investigation of CO catalytic oxidation by a Fe–PtSe2 monolayer
Abstract
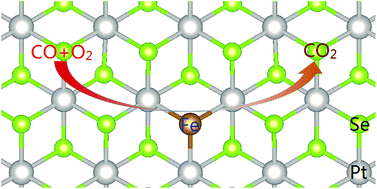
CO oxidation under mild conditions is investigated computationally for the catalysts based on a single transition metal (Sc–Zn) embedded at the Se vacancy of a PtSe2 monolayer. The iron-embedded Fe–PtSe2 monolayer is identified as the most suitable catalyst among the investigated systems. Both, Langmuir–Hinshelwood (LH) and Eley–Rideal (ER) reaction paths were considered for the CO oxidation by adsorbed O2 molecules and by adsorbed O atoms. The CO oxidation by O atoms bound to Fe–PtSe2 proceeds via the ER mechanism in a single reaction step with a small activation barrier (21 kJ mol−1). Both LH and ER reaction mechanisms can take place for CO oxidation by adsorbed O2 molecules. Whereas the barrier for the rate-determining step of the LH reaction path (72 kJ mol−1) is higher than that for the ER path (53 kJ mol−1), the kinetics analysis shows that both processes have comparable rate constants at 300 K. Langmuir–Hinshelwood mechanism becomes dominant at a lower temperature. Results reported here indicate that the Fe–PtSe2 catalyst can efficiently catalyze CO oxidation under mild conditions.
- This article is part of the themed collection: In memory of Petr Nachtigall


 Please wait while we load your content...
Please wait while we load your content...