Abstract
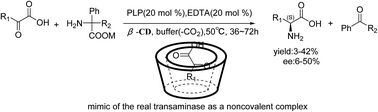
The mimics of vitamin B6-dependent enzymes that catalyzed an enantioselective full transamination in the pure aqueous phase have been realized for the first time through the establishment of a new “pyridoxal 5′-phosphate (PLP) catalyzed non-covalent cyclodextrin (CD)-keto acid inclusion complexes” system, and various optically active amino acids have been obtained.


 Please wait while we load your content...
Please wait while we load your content...