Tuning the solid-state fluorescence of chalcone crystals via molecular coplanarity and J-aggregate formation†
Abstract
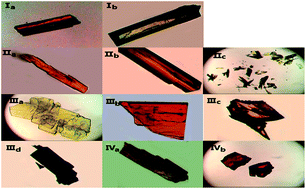
Four chalcones bearing the 1,3-diarylpropenone moiety 3-(9-anthryl)-1-(4-chloro)prop-2-en-1-one (I), 3-(1-pyrenyl)-1-(4-chlorophenyl)prop-2-en-1-one (II), 1-phenyl-3-(1-pyrenyl)prop-2-en-1-one (III) and 3-(1-pyrenyl)-1-(4-nitrophenyl)prop-2-en-1-one (IV) were synthesized and different crystal forms were obtained from different solvents by slow evaporation including I (Ia, Ib), II (IIa, IIb, IIc), III (IIIa, IIIb, IIIc, IIId) and IV (IVa, IVb). Their structures and optical properties were characterized by single crystal X-ray diffraction (SCXRD), powder X-ray diffraction (PXRD), thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), UV-Vis absorption spectroscopy and fluorescence spectroscopy. Hirshfeld surface calculations were also used for intermolecular interaction analysis. It has been found that polymorphism and conformational isomorphism exist in these crystals. Ib and IIIa adopt a cis configuration with respect to the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond while the other forms exhibit a trans configuration, except for those without single crystal X-ray analysis. Both Ib and IIb have two crystallographically independent molecules in their asymmetrical unit which has not been found in reported chalcone crystals. Those crystals with a cis configuration show a higher melting point and no fluorescence. Crystals with a trans configuration are fluorescent and their emission wavelengths are mainly effected by the torsion extent of the molecules and J-aggregate formation.
C bond while the other forms exhibit a trans configuration, except for those without single crystal X-ray analysis. Both Ib and IIb have two crystallographically independent molecules in their asymmetrical unit which has not been found in reported chalcone crystals. Those crystals with a cis configuration show a higher melting point and no fluorescence. Crystals with a trans configuration are fluorescent and their emission wavelengths are mainly effected by the torsion extent of the molecules and J-aggregate formation.


 Please wait while we load your content...
Please wait while we load your content...