Synthesis of di(ethylene glycol)-functionalized diketopyrrolopyrrole derivative-based side chain-conjugated polymers for bulk heterojunction solar cells†
Abstract
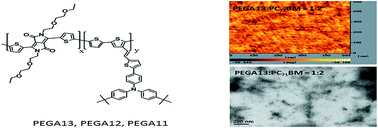
Polythiophenes (PTs) featuring di(ethylene glycol)-substituted 2,5-thienyl diketopyrrolopyrrole (DG-TDPP) moieties as conjugated units in the polymer backbone and tert-butyl-substituted triphenylamine (tTPA)-containing moieties as pendant units have been synthesized through Stille coupling. Incorporating the electron-deficient DG-TDPP moieties within the polymer backbone and appending the tTPA units promoted charge balance and efficient conjugation within the extended conjugated frameworks of the polymers, resulting in lower band gap energies and red-shifting the maximum UV-Vis absorption wavelength. The influence of the DG-TDPP content on the optical, electrochemical, and photovoltaic (PV) properties of the polymers has been studied. Incorporating a suitable content of DG-TDPP moieties in the polymer backbone enhanced the solar absorption ability and conjugation length of the PTs. The PV properties of bulk-heterojunction solar cells based on PT/fullerene derivatives improved after incorporating DG-TDPP units in the backbones of the side chain-conjugated PTs.


 Please wait while we load your content...
Please wait while we load your content...