High coercivity stellated cobalt metal multipods through solvothermal reduction of cobalt hydroxide nanosheets†
Abstract
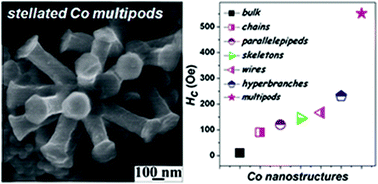
Solvated 2D nanosheets of dodecylsulphate intercalated α-cobalt hydroxide in 1-butanol are solvothermally reduced to hexagonal close packed (hcp) Co metal multipods in the presence of oleylamine. 1-Butanol, being a reducing solvent, facilitates the formation of Co metal. In addition to being a reducing agent, oleylamine also acts as a base which converts the metastable α-cobalt hydroxide nanosheets into β-hydroxide sheets. These sheets yield face centered cubic (fcc) CoO spheres which eventually grow into hcp Co metal multipods. All the pods are single crystalline with growth direction being [001], making the multipods resemble stellated polyhedra with overgrown branches. The stellated Co metal multipods exhibit high coercivity (Hc), of 880 and 552 Oe at 2 K and 300 K respectively, due to shape anisotropy.
- This article is part of the themed collection: 7th EuCheMS Chemistry Congress – Molecular frontiers and global challenges

 Please wait while we load your content...
Please wait while we load your content...