Synthesis of a biotinylated penta-α-(1→6)-d-glucoside based on the rational design of an α-stereoselective glucosyl donor†
Abstract
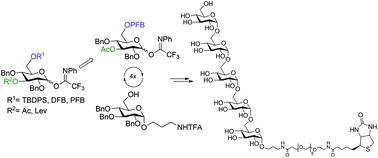
Two glucosyl donors, which allowed for the α-selective glucosylation of primary hydroxyl groups, were designed using a combination of acyl groups capable of remote participation, an electron-withdrawing pentafluorobenzoyl substituent and a bulky TBDPS protecting group. The first had a participating levulinoyl group at O-3 and the TBDPS protecting group at O-6, and the second bore an acetyl group at O-3 and the pentafluorobenzoyl one at O-6. With the help of these donors, two synthetic schemes, convergent and linear, towards a pentaglucoside representing the α-(1→6)-linked glucans of Helicobacter pylori were evaluated. The linear scheme proved to be preferential over the convergent one because of its insufficiently high α-stereoselectivity demonstrated by disaccharide donors. For the linear scheme, the pentafluorobenzoyl group turned out to be more convenient than TBDPS at the removal step. The α-(1→6)-linked pentaglucoside was prepared by iteration of glucosylation with the donor bearing 3-O-acetyl and 6-O-pentafluorobenzoyl and the subsequent liberation of the 6-OH group. Biotin was attached to the pentaglucoside via the spacer amino group.


 Please wait while we load your content...
Please wait while we load your content...