Synthesis of withasomnine and pyrazole derivatives via intramolecular dehydrogenative cyclization, as well as biological evaluation of withasomnine-based scaffolds†
Abstract
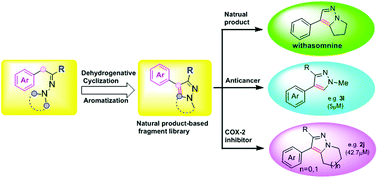
Fragment-based drug discovery has recently drawn great attention from both industry and academia. Fragment library design is critical to success in this field. Herein we report our facile access to pyrazole derivatives via a cascade reaction involving a double C(sp3)–H functionalization and dehydrogenative aromatization sequence. Based on the newly developed method, we completed the synthesis of the Indian herbal medicine, withasomnine, in only two steps. In addition, inspired by the different biological activities of withasomnine, this cascade reaction sequence enables us to quickly build up a natural product-based fragment library. The compounds in this fragment library exhibit a variety of biological activities and demonstrate high hit rates on initial biological screening against COX-2.


 Please wait while we load your content...
Please wait while we load your content...