Iridium catalyzed fragmentation/cyclization of N-butynyl 4,4-dimethylisoxazolidine-3,5-diones: a unique access to multiply substituted pyrroles†
Abstract
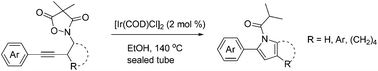
A unique protocol for the synthesis of substituted and fused pyrroles has been developed through the iridium catalyzed fragmentation/cyclization of related N-butynyl 4,4-dimethylisoxazolidine-3,5-diones in hot ethanol. Both the substitution pattern and electronic properties can affect the reaction rate and yield.


 Please wait while we load your content...
Please wait while we load your content...