Catalytic asymmetric C2-nucleophilic substitutions of C3-substituted indoles with ortho-hydroxybenzyl alcohols†
Abstract
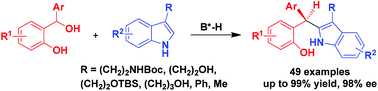
Catalytic asymmetric C2-nucleophilic substitutions of C3-substituted indoles such as tryptophols and tryptamines with ortho-hydroxybenzyl alcohols have been established, leading to the generation of triaryl methane products in generally high yields and excellent enantioselectivities (up to 99% yield, 98% ee). This class of C2-nucleophilic substitutions has wide substrate scope, which is not only applicable to a wide range of ortho-hydroxybenzyl alcohols, but also amenable to various tryptophols, tryptamines and other C3-substituted indoles. In addition, the reaction could be scaled up with maintained yield and enantioselectivity.
- This article is part of the themed collections: Celebrating Excellence in Research: 100 Women of Chemistry and Celebrating Excellence in Research: Women at the Frontiers of Chemistry


 Please wait while we load your content...
Please wait while we load your content...